Standard operating procedures (SOPs) and protocols are crucial for the effective management of laboratory work, providing reproducibility, efficiency, compliance, and safety.
However, when produced poorly or not followed correctly, protocols can also become a source of confusion, leading to resource waste, inaccurate results, and safety concerns.
This article takes a closer look at why SOPs are so important, and how you can use modern digital tools to save time and avoid mistakes.
What are SOPs and protocols?
The terms SOP and protocol are often used interchangeably, though these terms have slightly different meanings. In the laboratory environment, both SOPs and protocols are sets of instructions.
SOPs are used to describe more general lab methods, such as handling a particular hazardous substance or experimentation using a certain piece of equipment. Instructions might include recommended PPE, hazard control measures, transportation and storage, and waste disposal.
A protocol refers to detailed step-by-step instructions for a specific experiment, including the purpose, hypothesis, materials, methods, and other relevant information. Protocols may be used exclusively within a particular lab or shared with other laboratories.
SOPs provide instructions for routine tasks. Protocols are important in research laboratories where experimental procedures are developed and updated regularly.
How do you write a lab SOP?
An SOP is a formalized document that provides instructions so that staff knows how to carry out a given process. SOPs are often devised at the organizational level and distributed to various locations and departments. For example, universities typically have a library of SOPs outlining how to handle certain chemicals and use specific types of equipment.
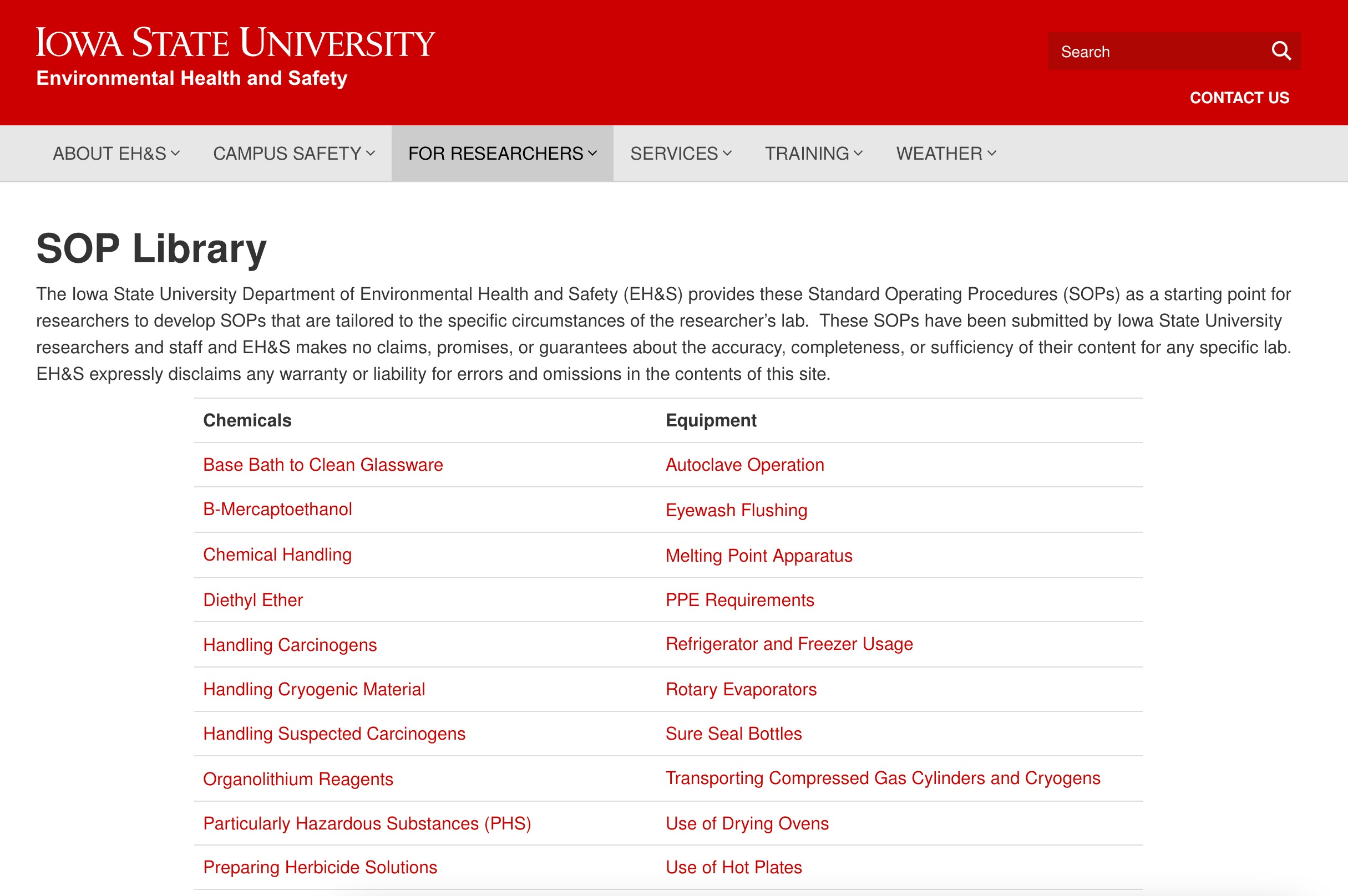
A snapshot of Iowa State University’s SOP library.
If you’re tasked with creating an SOP, here are some key pointers to help:
- Use a template: Starting with a template will help ensure your SOP has a consistent and logical structure. Find out if your organization or industry has templates or look to standard institutes such as ISO or the Clinical and Laboratory Standards Institute for guidance.
- Ensure the SOP can be practically followed: It’s easy to get bogged down in details and overlook major flaws in your procedure. Ask yourself obvious questions: Does your lab have all the appropriate equipment? Are the steps feasible within the timeframes specified?
- Ensure the SOP is legible, readable, and easy to comprehend: Printed SOPs must use an easily legible font face and size. The words and sentence structure should be easily readable, including bullets or numbered points, where applicable. The intended meaning of each sentence should be easy to understand with no ambiguity.
- Gain input from others: SOP writers should know the procedure well and garner input from staff following the instructions. Ideally, people unfamiliar with the procedure should also review the SOP for comprehensibility. The SOP must ultimately be approved by the lab manager or other superior (this may vary depending on the individual organization).
- Keep SOPs up to date and accurate: SOPs are living documents that need to be kept up to date. Have regulations changed? Has there been a change to the lab layout, equipment, or chemicals used? If so, staff must review all relevant SOPs and amend them accordingly. Keeping SOPs up to date will ensure compliance, improve quality and safety, and minimize errors.
How do you write a lab protocol?
One thing to bear in mind when writing a laboratory protocol is that at some point, the protocol may be utilized by different researchers in different locations. There should be no assumptions or areas that are open to interpretation. Someone in possession of an experiment protocol should be able to replicate the procedure exactly without asking for additional clarification.
Much of the above SOP advice applies when writing a protocol, including using templates and ensuring legibility and readability. Here are some additional points to consider:
- Include all major components: Each protocol should include purpose, materials (all reagents and equipment), methods (be specific, especially if there are multiple ways to do one thing), control information, data interpretation, and references.
- Document while you’re performing experiments: It’s surprising how quickly you can forget things after the fact, so it’s crucial to document every step and observation as you go.
- Use charts and diagrams liberally: Visuals such as diagrams and flow charts can be helpful for both comprehension and clarification of protocols. You may even want to incorporate videos. These should supplement the protocol rather than replace the text in case the reader has trouble viewing them.
- Document reagents in a standardized manner: Reagents can often be identified under multiple names or catalog numbers, so it’s important to avoid confusion by following standardized guidelines such as those provided by the Resource Identification Portal.
The Resource Identification Portal
What products and services exist to support lab protocols and SOPs?
Does the prospect of drafting an SOP or protocol from scratch sound stressful? Thankfully, there are plenty of tools available to help. Now that almost everything in the lab can be digitized, it is simple to work from existing templates or create your own. A good starting point is to look in repositories for shared experimental protocols such as protocols.io or Bio-Protocol.
If you use an electronic lab notebook, you may find that it offers SOP and protocol templates.
Colabra is ideal for collaborative lab protocols, as it offers built-in templates and versioning. Its mobile app is highly focused on facilitating teamwork and makes it easy to share your procedures with others.
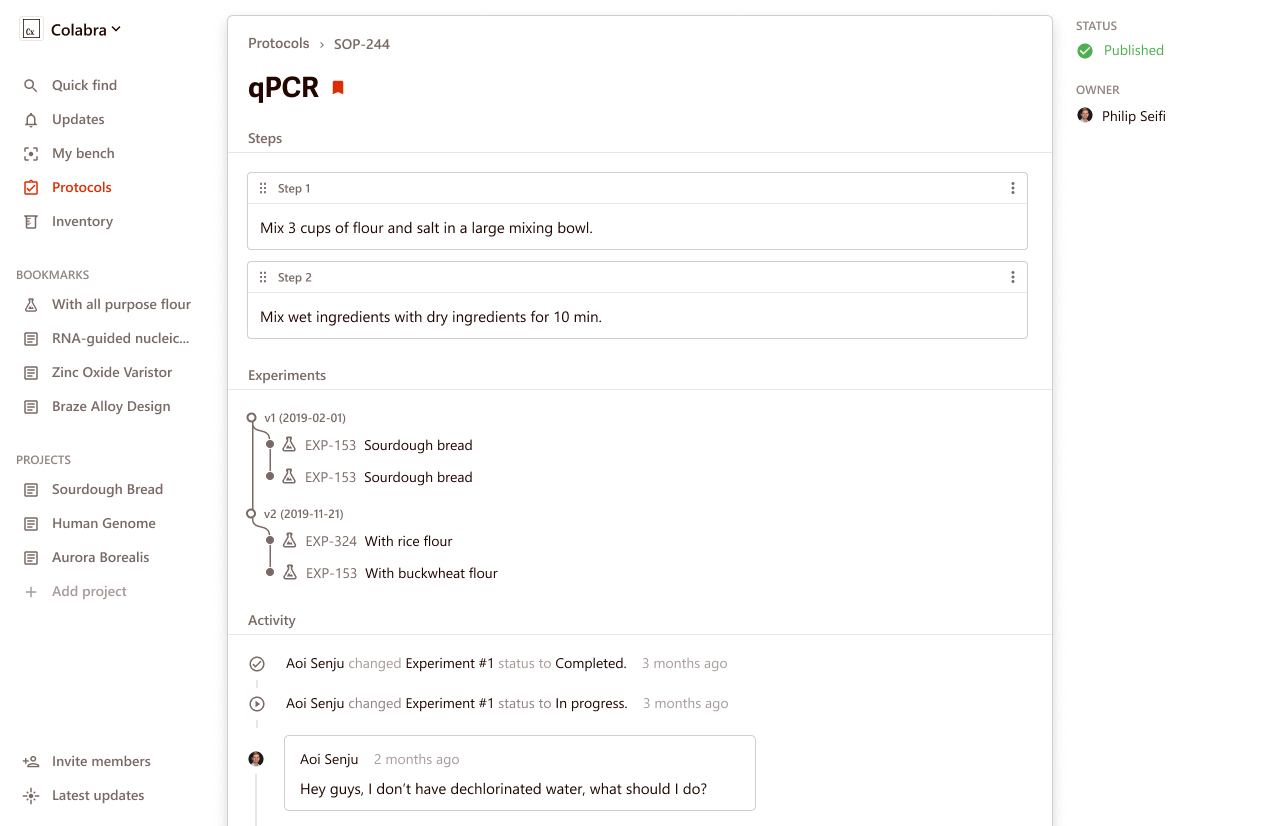
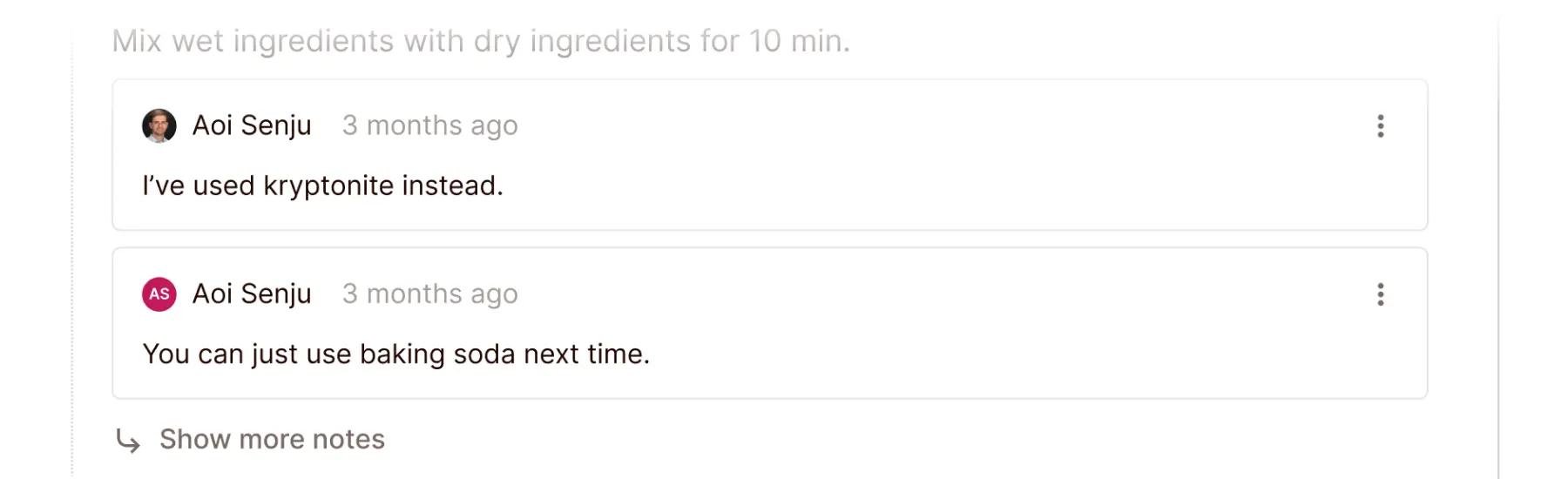
Colabra’s software even allows for communication between researchers within the protocol itself. For example, if a scientist has a specific question about a step in a protocol, they can ask it right on the protocol page.
Ready to digitize your SOPs?
SOPs and protocols are an essential part of any laboratory. By taking the time to establish lab protocols and SOPs, you can ensure that experiments are carried out safely and consistently, minimizing errors. There are many different products and services available to help with this process, so be sure to explore what’s out there to make your job a little easier.
If you'd like to learn more about how all this applies to your research, please shedule a Colabra demo, mention protocols, and we'll be glad to chat about the best ways to implement SOPs at your lab!

